CellEnvision comes from a team from Tsinghua University in Taiwan, with team members spanning the fields of biomedical, materials and clinical. With mature research and development capabilities for machines, reagents and detection platforms, it has successfully independently developed two core technology platforms: covering microfluidic chips, AI image recognition and high-throughput cell sorting. It is widely used in the cancer field and widely recognized by biomedical experts and cell therapy teams, and many long-term cooperative relationships have been successfully established.
Company Profile
Core tech

Provides services for the development of treatment strategies for various major diseases (such as cancer) with a world-leading microfluidic control combined with an open automated optical image analysis and cell culture platform in vivo single cell control.
Vision

Establish a liquid biopsy technology that can be dynamically monitored for a long time to find corresponding biological indicators of effective and ineffective treatment, so as to change the tumor immune microenvironment (TiME) created by tumors and achieve chronic cancer treatment.
Mission

The personalized medical liquid biopsy cell pathology database built with Tumor Immune Microenvironment (TiME) is planned to obtain global licensing of auxiliary diagnostic medical devices.
2012
- ● Core team established (National Tsing Hua University Biomedical Center Laboratory)
- ● Developed patented monolayer living cell SACA technology
2014
-
●
Preclinical trials for core technologies validation
(nearly 2,000 cases as of December 2024)
2019
- ● First-generation single-cell prototype developed
- ● Clinical trial collaboration: Combining CTCs and tumor markers to assess cancer recurrence accuracy in 179 colorectal cancer patients

2021
- ● Obtained ISO17025 laboratory certification
- ● Innovative discovery: CTMs can be found in the blood of cancer patients as an early detection marker for colorectal cancer recurrence
2022
- ● CellEnvision was established & obtained 2 instrument orders
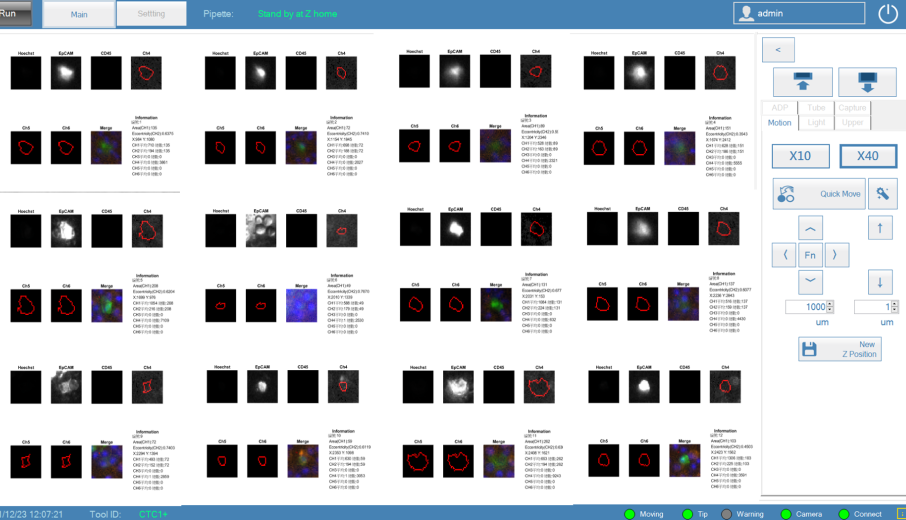
2024
- ● Clinical follow-up verification of multiple cancer types (breast cancer, lung cancer, and colorectal cancer) has been completed. CTM can not only be used as a recurrence indicator, but also the ratio and type of cancer cells and immune cells in CTM can be analyzed to evaluate the efficacy of medication

casee

cases